Introduction: Lung involvement in chronic graft versus host disease (cGVHD) is an uncommon but potentially devastating complication with significant morbidity and mortality in patients who have received an allogeneic hematopoietic stem cell transplant (alloHSCT). Unfortunately, early detection remains difficult as early lung cGVHD tends to be asymptomatic or present with nonspecific symptoms such as cough or mild shortness of breath. In British Columbia (BC), Canada, routine pulmonary function tests (PFTs) have been employed in the screening of lung cGVHD in this population at an interval of every 3-4 months for the first 2-3 years after alloHSCT. However, it is unknown whether routine screening 1) improves detection of lung cGVHD, and 2) enables earlier intervention to lead to better outcomes. We aimed to assess the real-world outcomes of lung cGVHD screening and compared the characteristics and clinical results of patients who had lung cGVHD detected via routine PFT screening, versus those who were detected after development of symptoms.
Methods: A retrospective chart review was conducted on all adult patients who underwent an alloHSCT at BC, Canada between June 2017 and January 2021. Patients were followed until August 2022. Patients were excluded if they had primary graft failure or if they died within 100 days after transplant. All types of pulmonary GVHD were included analysis and cases were established by the treating clinician in the clinical record. NIH consensus criteria were used to grade cGVHD severity. Statistical significance was calculated using the Chi square test for categorical variables and T-test for continuous variables.
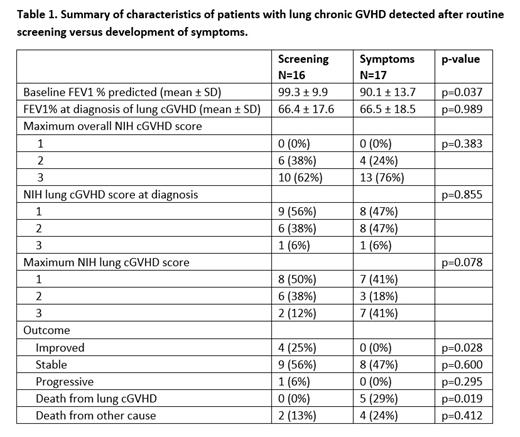
Results: Among 387 eligible consecutive patients who underwent alloHSCT, 189 (49%) developed cGVHD, with 33 (9%) cases of lung involvement. The mean time to diagnosis of lung cGVHD from the date of transplant was 13 ± 8 months. There were 16 cases of lung cGVHD (48%) that were initially detected by routine screening, while 17 cases (52%) were first detected due to clinical symptoms that developed in between screening visits or after the screening period ended (two patients developed symptoms at 27 months and 43 months respectively). Patient characteristics are presented on Table 1. Lung involvement was the first cGHVD sign in 17/33 (51%) of patients, with 5 (15%) detected by routine screening and 12 (36%) due to symptoms. At time of diagnosis of lung involvement, 12/33 (36%) of patients were already on systemic immunosuppression for cGVHD with 9 (56%) of these cases detected from screening and 3 (18%) from symptoms (p=0.02). All patients had a maximum overall cGVHD severity by NIH criteria of 2-3. Sixteen of 33 (48%) patients had a lung cGVHD score by NIH criteria of 2-3 at diagnosis, with a mean FEV1 of 54% ± 15, compared to 17 (52%) patients with a score of 1 and mean FEV1 of 78% ± 12. The NIH score at diagnosis was not significantly different between those picked up by routine screening versus symptoms (p=0.76). Eighteen of 33 (55%) patients had a maximum lung cGVHD score by NIH criteria of 2-3, with the mean lowest FEV1 of 52% ± 15, compared to 15 patients (45%) with a maximum lung cGVHD score of 1, with the mean lowest FEV1 of 77% ± 10. Similarly, the maximum NIH lung cGVHD score were not significantly different between those picked up by routine screening versus symptoms (p=0.08). However, patients picked up on screening were more likely to have significant clinical improvements in lung function (4/16 [25%] versus 0/17 [0%], p=0.03), and less likely to die from lung cGVHD (0/16 [0%] versus 5/17 [29%], p=0.02).
Conclusions: Routine screening PFTs are effective for early diagnosis of asymptomatic lung cGVHD, and are associated with improved outcomes compared to patients who were diagnosed after symptoms developed. Since a significant number of these patients were not previously receiving immunosuppression for other cGVHD, this suggests a potential benefit in screening all patients every 3 months, regardless of prior GVHD status.
Disclosures
Mourad:Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Honoraria, Speakers Bureau; Alexion: Consultancy; Paladin: Honoraria, Speakers Bureau. Chung:Takeda: Consultancy, Honoraria; Astella Pharma: Honoraria; Novartis: Honoraria; Paladin: Honoraria. Sanford:Astellas: Honoraria; AbbVie: Honoraria. Song:Novartis: Honoraria; Amgen: Honoraria; Sanofi: Honoraria; Janssen: Honoraria; GSK: Honoraria; BMS: Honoraria; Forus: Honoraria; Gilead: Honoraria. Stubbins:AbbVie: Consultancy, Honoraria; Jazz Pharmaceuticals: Honoraria; Pfizer: Honoraria. Toze:AbbVie: Honoraria, Research Funding; Beigene: Honoraria; Janssen: Honoraria; Astra-Zeneca: Research Funding. White:Novartis: Honoraria. Hay:Novartis: Honoraria; Kite/Gilead: Honoraria; BMS: Honoraria; Janssen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal